
The Meaning of Care Magazine
‘Heart and Soul’: Innovative, Patient-Centered Care Leads to Cardiac Clinical Trial Excellence
Published: Aug. 24, 2021After receiving another compliment on his tropical-print shirt, Pat Thomas revealed the story behind it.
“Every Friday was Hawaiian shirt day at work,” the retired Omaha Public Power District database manager said. “So, I guess you could say I’ve got a rather nice collection.”

His dry humor was on full display during a postoperative appointment in May, but it suddenly ceased when trying to explain his gratitude for a clinical trial he’s currently enrolled in.
He pressed his lips together and nodded as the realization hit him: This trial may have given him many more days to sport those bright floral shirts. Better yet, it may have given him the energy to say yes to more walks with his wife, more touch football with his grandson and more lawnmower rides with his granddaughter.
“They’re little things,” he said as he wiped away a tear. “But they’re good things.”
Innovative Care
The clinical research coordinators at Methodist Physicians Clinic – Dru McMartin, CCRC, MA, and Danielle Frodyma, PhD – spend a lot of time with Pat, coordinating his care and serving as extra sets of eyes during his follow-up visits. So, they know a lot about him, including his real name. To help prevent research bias and uphold privacy laws, Pat’s real name and age must be kept from EBR Systems Inc. – the makers of an innovative device that’s tucked deep inside his chest.
With the help of McMartin and Frodyma, EBR Systems Inc. is currently studying Pat and his response to its WiSE™ CRT (cardiac resynchronization therapy) System, which provides leadless pacing for heart failure patients.
Methodist electrophysiologist Matthew Latacha, MD, has spent years researching the technology, as he’s fully aware of the problems that leads – or wires that are run through the heart – can cause with traditional CRT devices.
“We see so many issues with them,” Dr. Latacha said. “Infections, dislodgement, fractures. So, to get away from any kind of leads is an ideal situation.”
The WiSE™ CRT System involves the implantation of an ultrasound transmitter, which is placed between the ribs. That transmitter sends an ultrasonic pulse through the chest, to a tiny electrode (about the size of a cooked grain of rice) that’s implanted inside the heart.
“Every time the heart beats, that electrode is stimulated and causes a pacing stimulus in the left part of the heart, resynchronizing the heart,” Dr. Latacha said. “Just like a regular lead would.”
Despite the traditional pacemaker that Pat’s had for years and the various medications he’s tried, his heart was still beating out of sync, causing him chronic fatigue, shortness of breath and a decreased life expectancy.
Dr. Latacha knew this made Pat – his patient of seven years – the perfect candidate for the WiSE™ CRT clinical trial (SOLVE-CRT). So, he proposed it to him, sparing no details about what the study at Methodist would involve.
“One way or another, I was going to need something done to my heart,” Pat said. “And this seemed like it might be the best option. Of course, I didn’t have many options left.”
Relationship-Based Enrollment
In May, Dr. Latacha implanted Pat’s WiSE™ CRT device as representatives of EBR Systems Inc. supervised his every move.
“There are very few sites in the U.S. that were chosen to do this,” Dr. Latacha said. “There was a pretty extensive screening process that we had to go through. When I got the call from the company – saying, ‘Hey, do you want to be part of the trial?’ – I was ecstatic. And I think they were, too. They were excited about working with the lab and our research staff.”

Frodyma, who’s new to Methodist but no stranger to clinical research, said she was immediately attracted to the health system’s patient-centered approach in trials such as SOLVE-CRT.
“This is a hospital,” she said. “Here, it’s just about patient care and giving them the best unbiased options possible. Nobody’s looking to publish any sort of academic paper. They don’t have their own agenda tied to a specific grant.”
“We care,” added McMartin, who helped shape Methodist into a preferred provider for several drug and device companies when she joined the clinical research team in 2015. “We’re The Meaning of Care. It’s not about the money or the numbers for us. We want someone else’s mom, someone else’s son or someone else’s best friend to be safe, healthy and well. We treat them like individuals instead of numbers.”
As a result, Methodist’s cardiac, vascular and pulmonary clinical trial team, which currently manages 13 active studies with more than 40 enrolled participants, hasn’t lost one patient to follow-up since 2015. In other words, not one enrollee has chosen to discontinue care – or cancel their follow-up visits – after committing to a study. According to McMartin, that’s what yields good data and makes the health system so attractive to clinical research organizations charged with selecting the best, most reliable sites for their studies.
“That’s an issue for a lot of sites doing cardiac clinical trials,” Dr. Latacha said. “Other sites may have a lot of clinical trials, but they can’t enroll patients or keep them enrolled because they don’t have the same relationship with their patients that we have with ours.”
Steve Sewell, a field engineer for EBR Systems Inc., agrees. The Meaning of Care, he said, is just the beginning of why Methodist was chosen for the SOLVE-CRT study.
“We work all over the country,” he said. “The thing I like about Methodist is the feel – the atmosphere. The equipment is nice. The rooms are spacious. Dr. Latacha is very passionate about this procedure, and the staff is just fantastic. But the passion for the patients – making sure we get the best candidates and that they get the best outcomes – that’s what stands out.”
And thanks to Methodist’s dedication in landing the study, Pat has been given an 85% chance of improved heart function – and, hopefully, a longer life.
“That’s a chance he might not have otherwise had,” Dr. Latacha said.
“Future of the Field”
Two days after his procedure, Pat noticed his shortness of breath had almost completely disappeared. He felt “functional” again. It was the first time he felt the same kind of excitement that Dr. Latacha has long expressed.
“You want somebody like him because when you’re enthusiastic about something, you’re going to throw your heart and soul into it,” Pat said, placing his hand on his Hawaiian-covered chest. “You’re going to give it all you can. And with the care I’ve received at Methodist, everyone has. So, this has been … a very good experience. I’d recommend it to the world.”
Like many other clinical trials at Methodist – including those related to cancer, infectious disease and other conditions – it could be years before all follow-up of SOLVE-CRT is complete. But according to Dr. Latacha, the hope of better outcomes for all is well worth the wait.
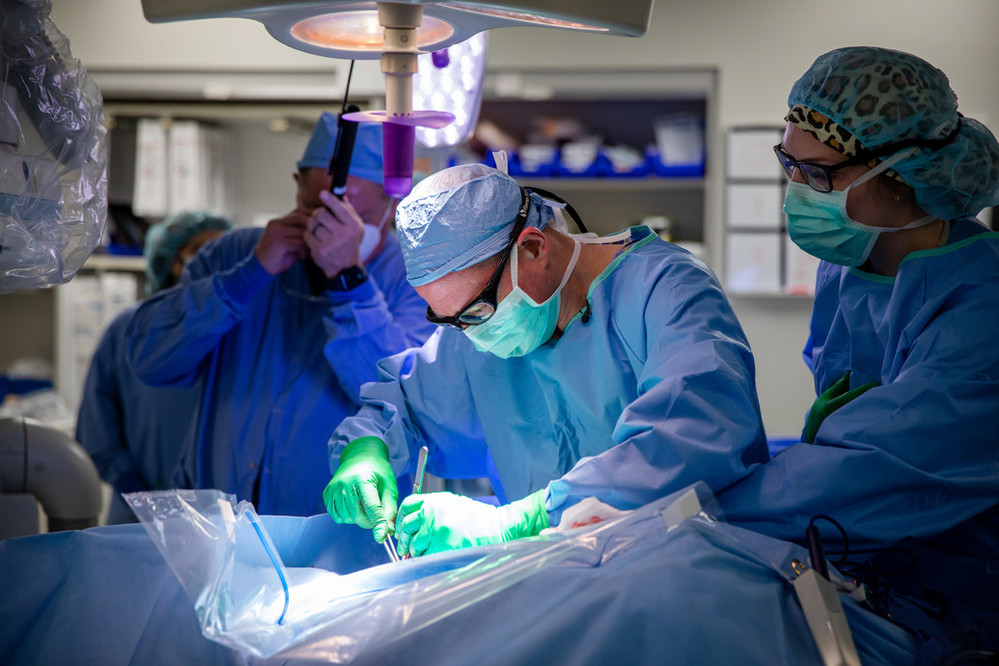
“It’s remarkable,” he said. “This is the future of the field. I think this is what we’re looking at for this patient population in the next 10 years or so, and we can say that we were one of the first centers in the country – in the world, even – to do this.”
More Resources
- Read more from the summer 2021 issue of The Meaning of Care Magazine.
- Learn more about clinical trials at Methodist.
- Learn more about cardiac specialties and services at Methodist.
- Learn more about the WiSE CRT System.


